Product Design
MEDICAL–LEGAL–REGULATORY (MLR) REVIEW PLATFORM
An automated review tool designed to reduce manual effort, compliance risk, and approval delays in healthcare marketing.
My Role :
0-1 Product Designer
Industry :
Pharma / Healthcare (B2B)
Year(s) :
2020-2021
Project Duration :
~ 1 year
Tools :
Adobe Creative XD, Sketch, Mural, JIRA and Hotjar,



Project Overview
The Medical, Legal, and Regulatory (MLR) Review is essential in healthcare marketing to ensure accurate, compliant content ready for publication. In industries like pharmaceuticals and biotech, misinformation can cause serious harm, fines, and damage to reputation. Traditionally, MLR reviews are manual, involving emails and spreadsheets, which lead to inefficiencies and delays. This project proposes an automated MLR tool to improve accuracy, cut costs, streamline processes, and speed product releases. The COVID pandemic and remote work in 2020 highlighted these issues, underscoring the need for a centralized system to reduce manual efforts and accelerate compliant approvals.
So why do we need MLR?








Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
Research
Research Methods
Domain exploration: Understanding MLR standards, review stages, and compliance constraints
Stakeholder interviews: Regulatory specialists/SMEs + cross-functional reviewers (Medical, Legal, Marketing)
Competitive analysis: Reviewed existing tools (e.g., Veeva Vault PromoMats) to identify patterns and gaps
Concept validation: Early concepts reviewed with SMEs to confirm feasibility and adoption barriers
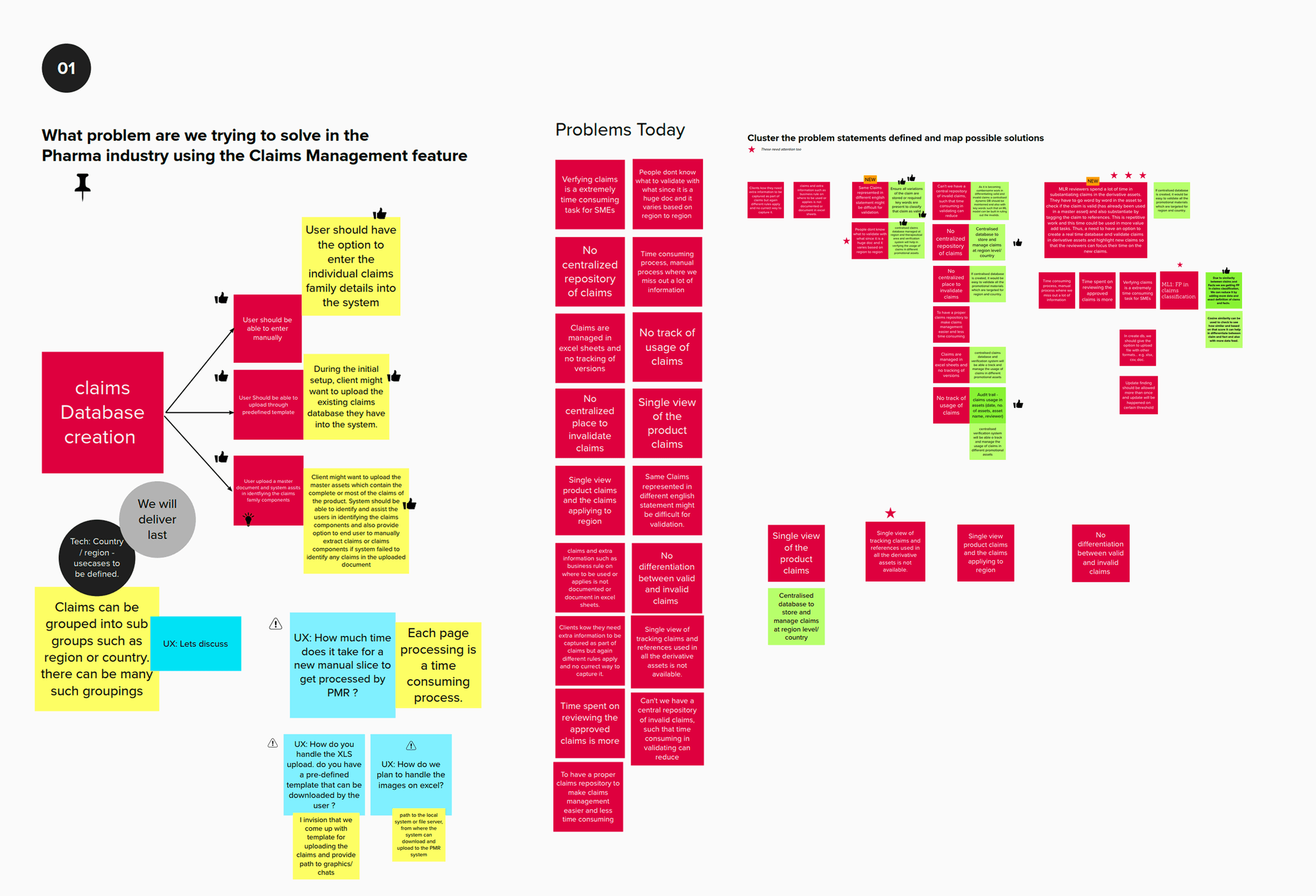
Pain points
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
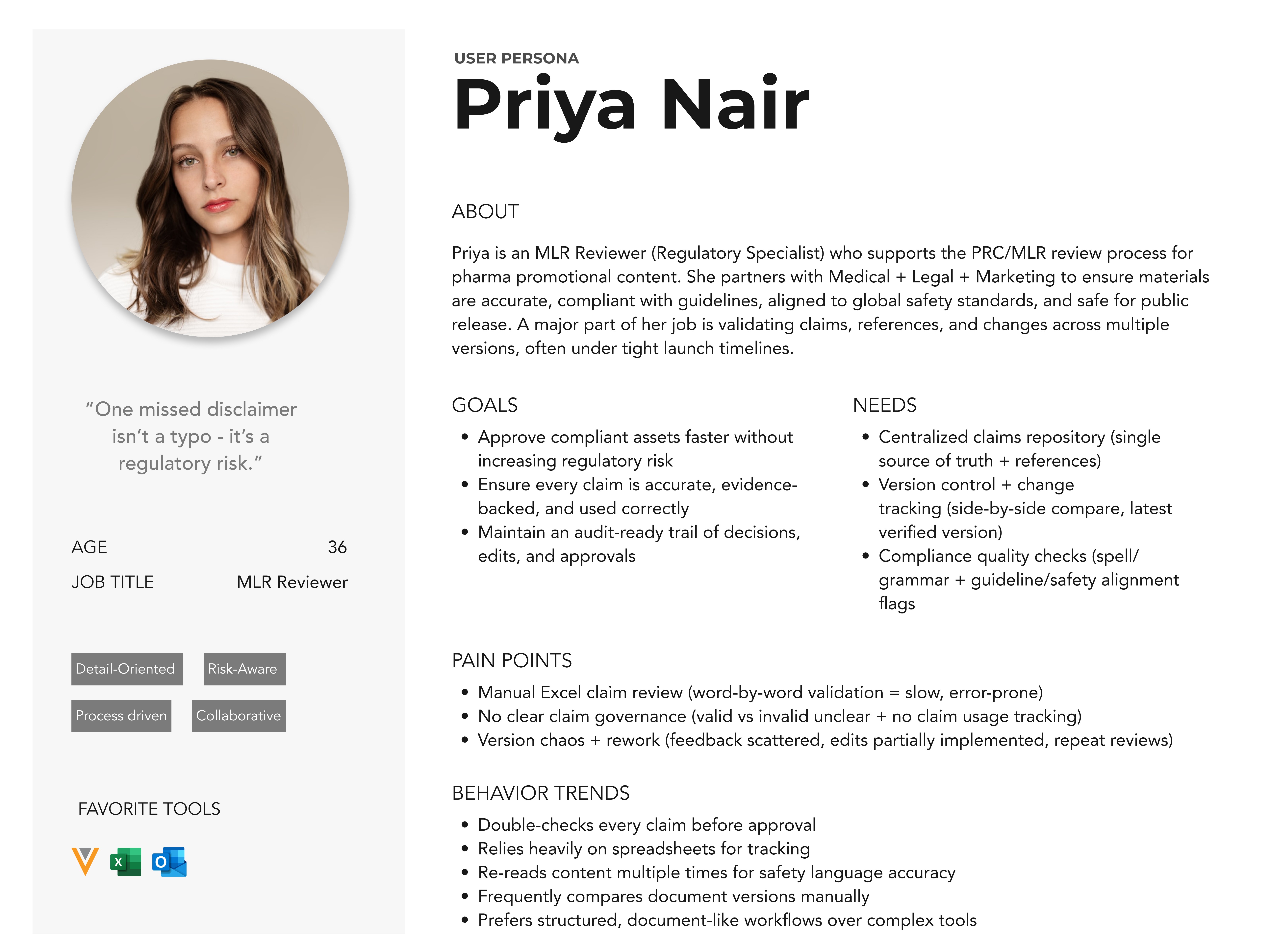
Persona
Design Thinking session + user requirement gathering



Features
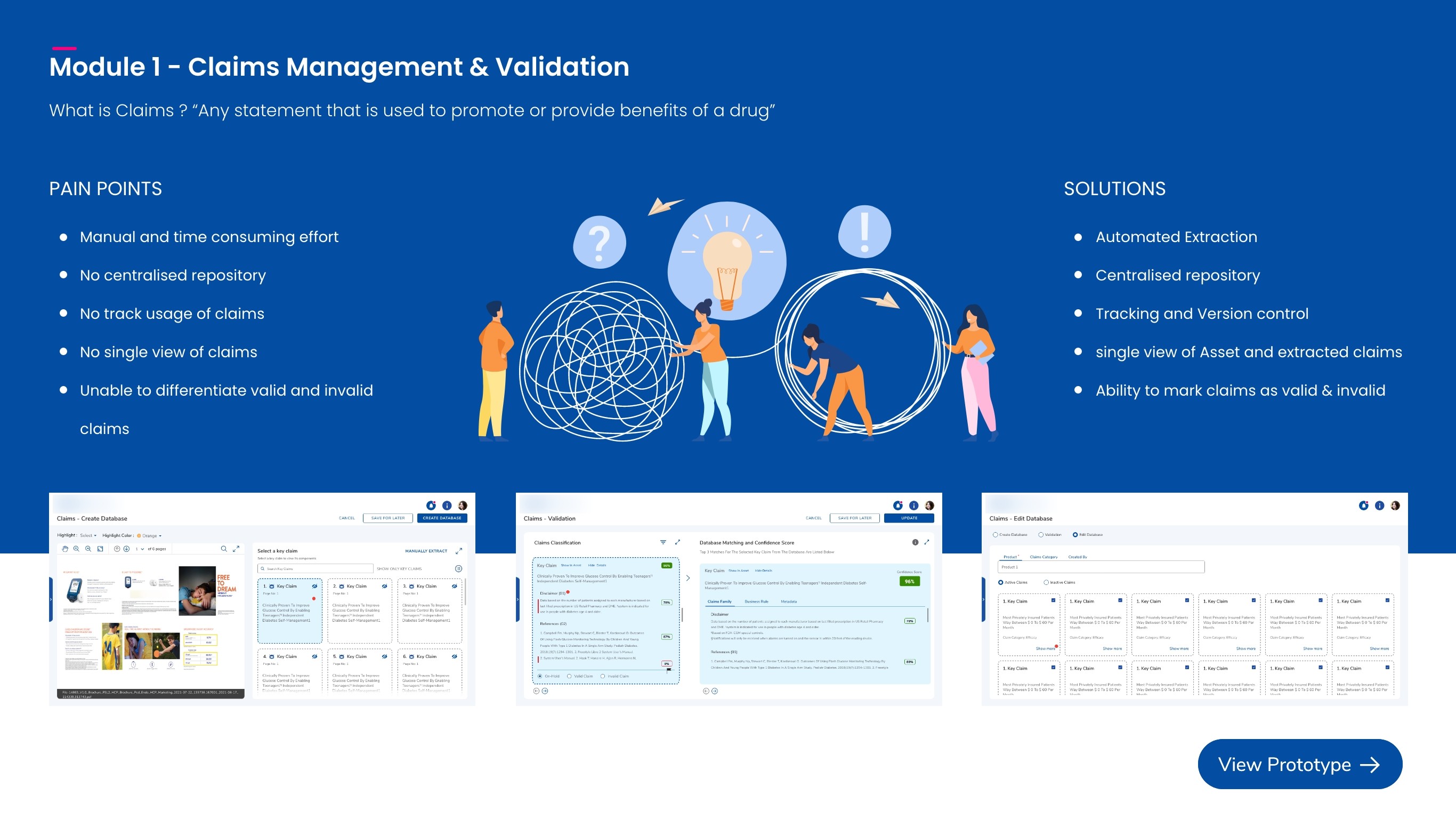
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
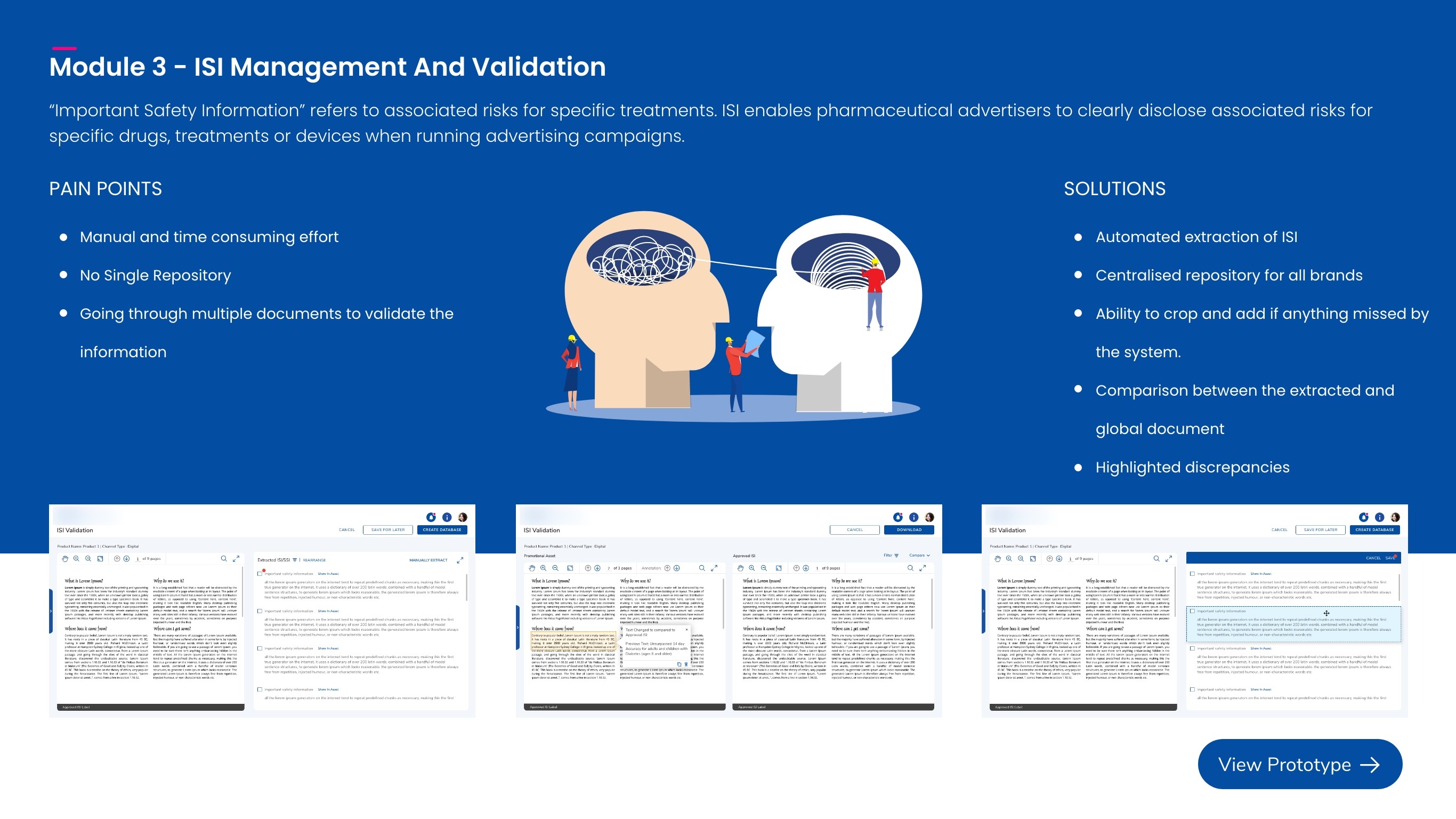
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
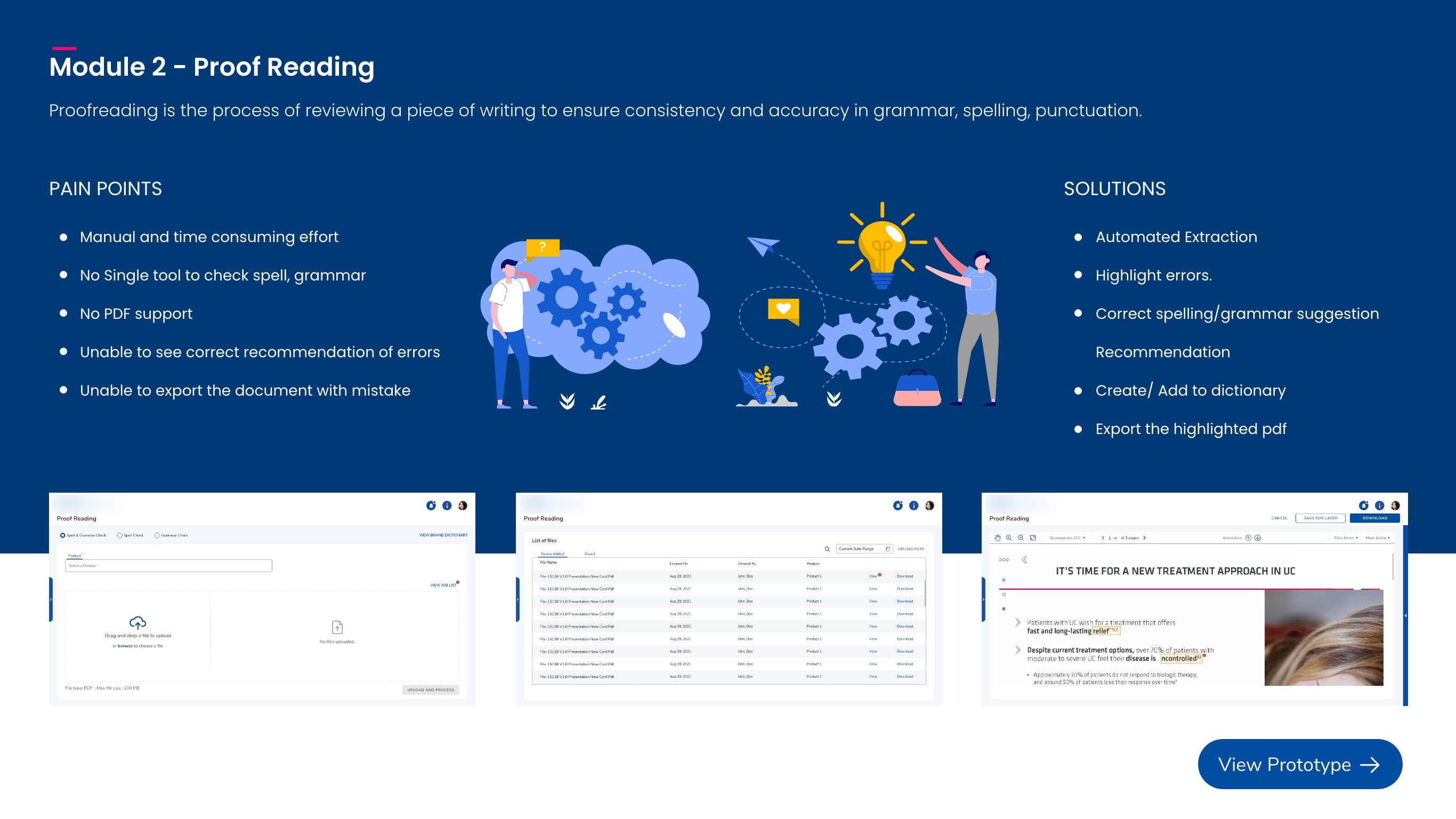
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
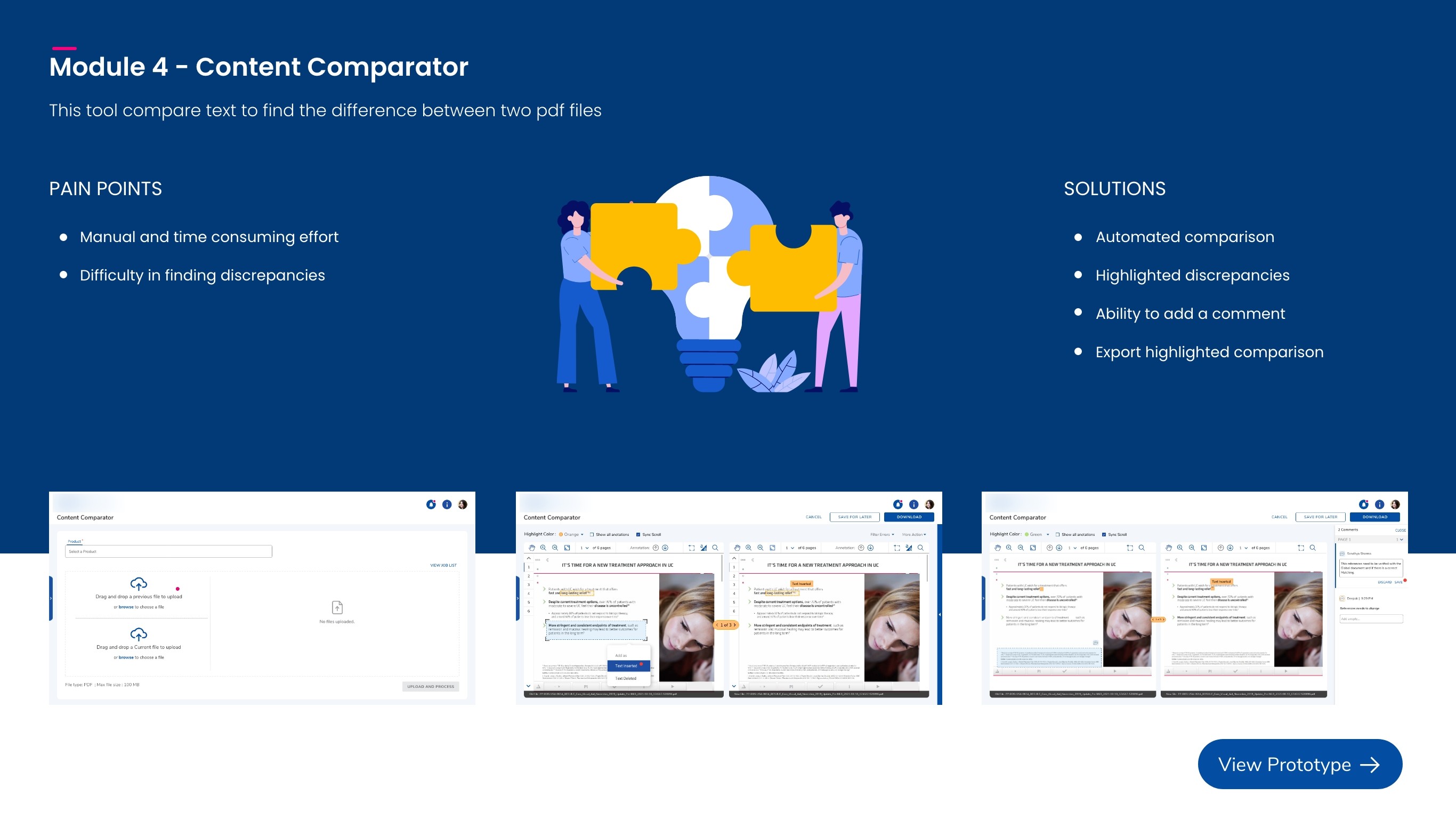
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Design
Wireframe


First Design Draft
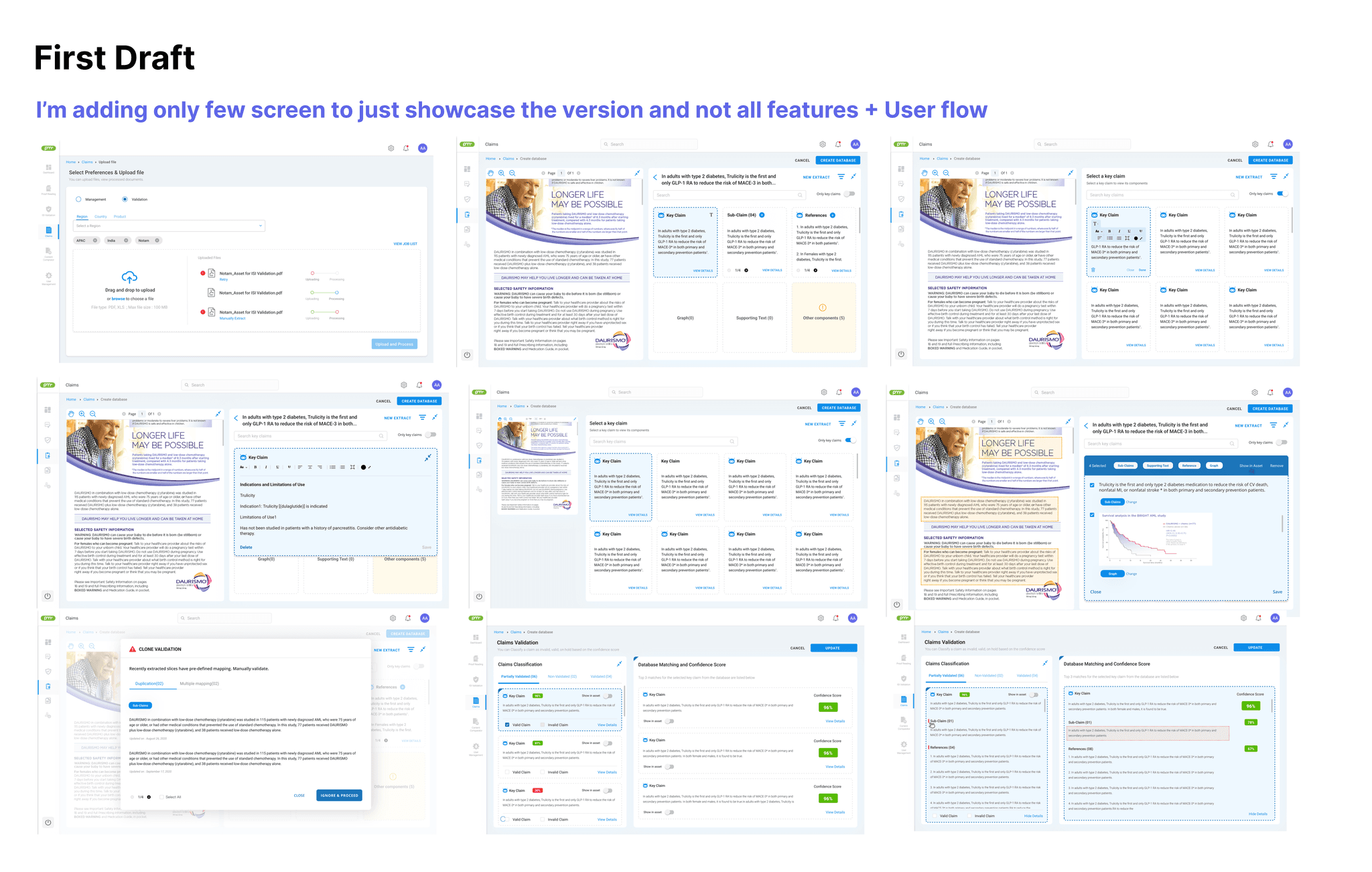

In the first draft, my goal wasn’t to force reviewers into a new way of working, it was to fit into how MLR teams already operate. I leaned on familiar, document-like interactions (text selection, keyboard behavior, and comment-style feedback) so the experience felt intuitive from day one and required minimal training.
I also focused on reducing repetitive work, especially cropping the same claim repeatedly across assets and versions. To streamline this, we supported both auto-cropping and manual cropping when needed. For manual capture, I added clone-validation warnings to flag duplicate claims and prevent rework. This helped reviewers spend more time on compliance judgment, not repetitive claim extraction.
Feedback
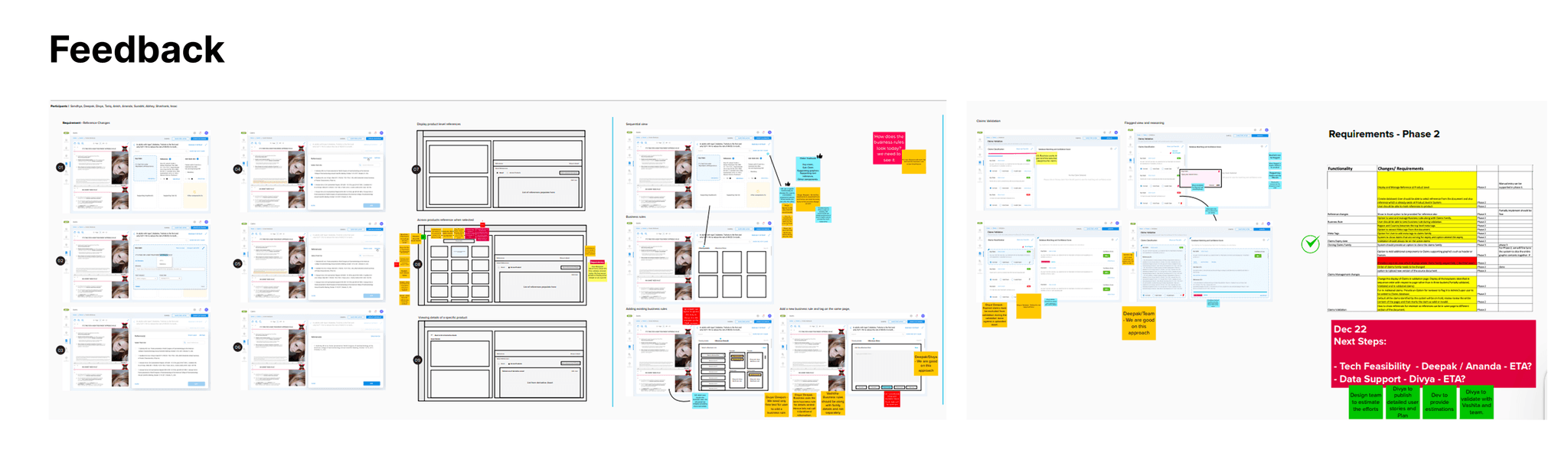

Final Version
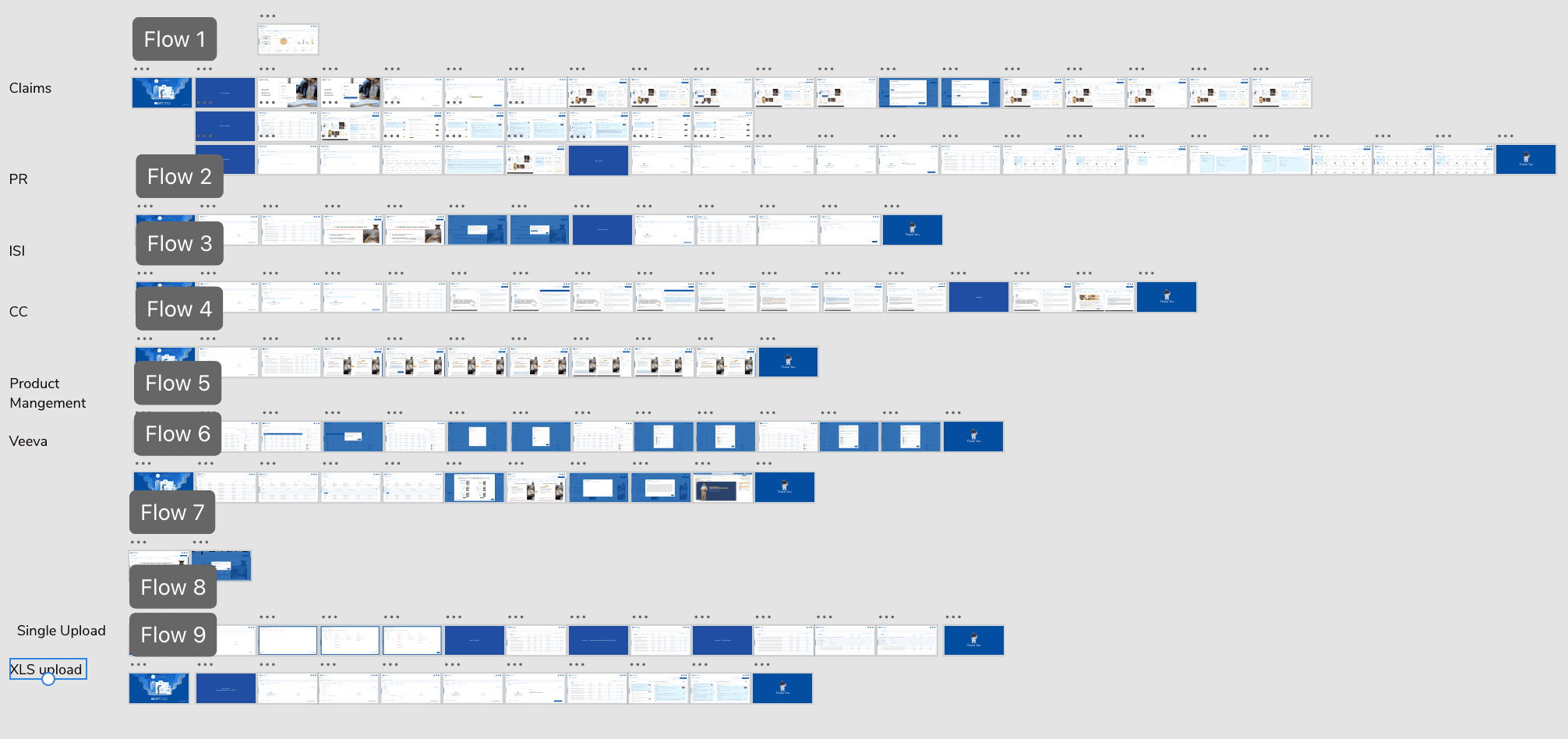

Outcome
Over ~1 year, we transformed a fragmented, manual MLR workflow into a centralized review platform shortening approval timelines by up to 40%, reducing duplicate claim-related rework by ~30%, and significantly improving audit readiness through clear version tracking and structured approval states.
Key Learnings
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
Product Design
MEDICAL–LEGAL–REGULATORY (MLR) REVIEW PLATFORM
An automated review tool designed to reduce manual effort, compliance risk, and approval delays in healthcare marketing.
My Role :
0-1 Product Designer
Industry :
Pharma / Healthcare (B2B)
Year(s) :
2020-2021
Project Duration :
~ 1 year
Tools :
Adobe Creative XD, Sketch, Mural, JIRA and Hotjar,



Project Overview
The Medical, Legal, and Regulatory (MLR) Review is essential in healthcare marketing to ensure accurate, compliant content ready for publication. In industries like pharmaceuticals and biotech, misinformation can cause serious harm, fines, and damage to reputation. Traditionally, MLR reviews are manual, involving emails and spreadsheets, which lead to inefficiencies and delays. This project proposes an automated MLR tool to improve accuracy, cut costs, streamline processes, and speed product releases. The COVID pandemic and remote work in 2020 highlighted these issues, underscoring the need for a centralized system to reduce manual efforts and accelerate compliant approvals.
So why do we need MLR?









Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
Research
Research Methods
Domain exploration: Understanding MLR standards, review stages, and compliance constraints
Stakeholder interviews: Regulatory specialists/SMEs + cross-functional reviewers (Medical, Legal, Marketing)
Competitive analysis: Reviewed existing tools (e.g., Veeva Vault PromoMats) to identify patterns and gaps
Concept validation: Early concepts reviewed with SMEs to confirm feasibility and adoption barriers
Pain points
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Persona
Design Thinking session + user requirement gathering




Features
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Design
Wireframe


First Design Draft


In the first draft, my goal wasn’t to force reviewers into a new way of working, it was to fit into how MLR teams already operate. I leaned on familiar, document-like interactions (text selection, keyboard behavior, and comment-style feedback) so the experience felt intuitive from day one and required minimal training.
I also focused on reducing repetitive work, especially cropping the same claim repeatedly across assets and versions. To streamline this, we supported both auto-cropping and manual cropping when needed. For manual capture, I added clone-validation warnings to flag duplicate claims and prevent rework. This helped reviewers spend more time on compliance judgment, not repetitive claim extraction.
Feedback


Final Version


Outcome
Over ~1 year, we transformed a fragmented, manual MLR workflow into a centralized review platform shortening approval timelines by up to 40%, reducing duplicate claim-related rework by ~30%, and significantly improving audit readiness through clear version tracking and structured approval states.
Key Learnings
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
Product Design
MEDICAL–LEGAL–REGULATORY (MLR) REVIEW PLATFORM
An automated review tool designed to reduce manual effort, compliance risk, and approval delays in healthcare marketing.
My Role :
0-1 Product Designer
Industry :
Pharma / Healthcare (B2B)
Year(s) :
2020-2021
Project Duration :
~ 1 year
Tools :
Adobe Creative XD, Sketch, Mural, JIRA and Hotjar,



Project Overview
The Medical, Legal, and Regulatory (MLR) Review is essential in healthcare marketing to ensure accurate, compliant content ready for publication. In industries like pharmaceuticals and biotech, misinformation can cause serious harm, fines, and damage to reputation. Traditionally, MLR reviews are manual, involving emails and spreadsheets, which lead to inefficiencies and delays. This project proposes an automated MLR tool to improve accuracy, cut costs, streamline processes, and speed product releases. The COVID pandemic and remote work in 2020 highlighted these issues, underscoring the need for a centralized system to reduce manual efforts and accelerate compliant approvals.
So why do we need MLR?









Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Problem
The traditional MLR review process is inefficient, error-prone, and costly, posing significant risks to pharmaceutical, biotech, and life sciences companies.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
Goal
Design and deliver a centralized, automated MLR review tool that replaces manual email/spreadsheet workflows, reducing compliance risk and rework while speeding up traceable, cross-functional approvals and time-to-market.
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
My Responsibilities
Conducting research (domain understanding, stakeholder/SME interviews, competitive scan)
Design thinking workshop to translate business requirements into user flows and information architecture
Creating wireframes to explore solutions quickly
Iterating on designs based on stakeholder feedback and usability findings
Building high-fidelity prototypes to validate end-to-end workflows before development
Partnering with cross-functional teams (Medical, Legal, Regulatory, Marketing, Product) to align on constraints, approvals, and launch readiness
Research
Research Methods
Domain exploration: Understanding MLR standards, review stages, and compliance constraints
Stakeholder interviews: Regulatory specialists/SMEs + cross-functional reviewers (Medical, Legal, Marketing)
Competitive analysis: Reviewed existing tools (e.g., Veeva Vault PromoMats) to identify patterns and gaps
Concept validation: Early concepts reviewed with SMEs to confirm feasibility and adoption barriers
Pain points
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Manual coordination → delays & rework
Email/spreadsheet-based reviews caused repeated back-and-forth, miscommunication, and extra review cycles.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Version confusion → compliance risk
Teams lacked a clear “single source of truth,” making it easy to lose track of the latest approved file and what changed.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Repetitive validation work → reviewer fatigue
Claim checks, reference matching, and ISI consistency reviews were highly manual and time-consuming, pulling reviewers away from higher-value judgment.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Tool fragmentation → poor collaboration
Switching across multiple systems (docs, spreadsheets, review tools) broke context, slowed approvals, and increased the chance of missed or inconsistent updates.
Persona
Design Thinking session + user requirement gathering




Features
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Claims Management
Prevents duplicate claim capture with clone-validation errors and matches cropped claims to a database with confidence scores to quickly label them as valid/invalid and speed up review.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Important Safety Information(ISI)
Validates each asset’s safety block against a region-specific ISI database (e.g., FDA/EMA/CDSCO) to ensure the correct, up-to-date safety language is used.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Proof Reading
Scans PDFs to flag spelling and grammar issues while accounting for medical terminology that still requires reviewer vetting.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Content Comparator
Compares the latest asset to the previous version to highlight what changed so reviewers can quickly verify updates and reduce rework.
Design
Wireframe


First Design Draft


In the first draft, my goal wasn’t to force reviewers into a new way of working, it was to fit into how MLR teams already operate. I leaned on familiar, document-like interactions (text selection, keyboard behavior, and comment-style feedback) so the experience felt intuitive from day one and required minimal training.
I also focused on reducing repetitive work, especially cropping the same claim repeatedly across assets and versions. To streamline this, we supported both auto-cropping and manual cropping when needed. For manual capture, I added clone-validation warnings to flag duplicate claims and prevent rework. This helped reviewers spend more time on compliance judgment, not repetitive claim extraction.
Feedback


Final Version


Outcome
Over ~1 year, we transformed a fragmented, manual MLR workflow into a centralized review platform shortening approval timelines by up to 40%, reducing duplicate claim-related rework by ~30%, and significantly improving audit readiness through clear version tracking and structured approval states.
Key Learnings
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
Takeaway
Designing for familiarity drives adoption.
By using document-like interactions and reducing repetitive claim cropping, we lowered resistance to change and improved reviewer confidence.
Compliance workflows require trust, not just speed.
Version clarity, audit trails, and duplicate claim validation were critical to reducing risk while increasing efficiency.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.
What I learnt?
Even small workflow improvements (like preventing duplicate claim capture) can meaningfully reduce review fatigue. Introducing UX to a non-design team requires advocacy, alignment, and demonstrating how design decisions directly reduce business risk.
In regulated industries, success isn’t just usability, it’s building confidence in compliance.